
Ever since K. Eric Drexler published his
1987 nonfiction book Engine of Creation, science fiction has
been alive with speculation about "nanotechnology"--the future
construction and application of molecule-sized machinery capable of
manipulating matter at the level of individual atoms. One of the
ideas Drexler proposed was "smart materials" made of tiny,
programmable machines rather than normal, inert molecules. To some
extent, a laptop computer's liquid crystal display (LCD) meets this
definition, but Drexler's proposal included future advances such as
"paint" which could spread itself automatically, change color and
texture on command, etc., and even "smart walls" in which the
windows and doors could be moved around as easily as picture frames.
Still, science fiction often falls
back on nanotechnology as an almost magical plot device, capable not
only of changing texture or shape, but of building permanent
structures from "seeds" and adding exponentially (or even instantly)
to its own substance. While in principle this may be possible, the
nanomachines which make up "smart matter" will need an energy source
(probably specialized chemical fuels) and a source of raw materials
(probably specialized chemical feedstocks), so as in biology,
delivery and distribution of "nutrients" becomes a primary function
of the system. Any other activity has to take a lower priority,
fitting in where time and resources permit.
If nanotech applications were needed
outside the laboratory, they'd have to resemble plants or molds,
drawing energy and nutrients from the immediate environment (perhaps
through elaborate systems of "leaves" or "roots") in order to feed
themselves. In nature, these metabolic processes are slow, although
bamboo and mushrooms can sometimes grow at naked-eye speeds, and a
typical acre of forest or grassland does contain a great deal of
harvestable chemical energy, as the rapid release of a brush or
forest fire will demonstrate. But while it may be possible to build
nanomachines with faster-than-natural metabolism, this heightens the
danger of a runaway replication or "gray goo" or "bloom" event--the
ultimate nanotech nightmare.
Utility fog may free us all
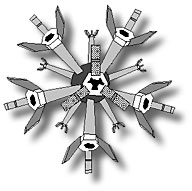
With such difficulties in mind, Dr.
J. Storrs Hall of Rutgers University built on Drexler's concepts in
the early 1990s by proposing a "utility fog" of 12-armed, dust-sized
silicon micromachines ("foglets," as seen at right in an image
courtesy of J. Storrs Hall) capable of joining hands in a variety of
configurations to create a programmable substance which could assume
any shape, and vary its density from that of vapor or cobwebs to
something as solid as wood, plastic or even porous cement. This is
not only the ultimate modeling clay and 3-D entertainment system (holodeck,
anyone?), but potentially an important structural component, safety
system and furnishing for homes, factories and vehicles.
Utility fog is a more achievable
technology than Drexler's nanotech, since its key components are
roughly a thousand times larger, and since primitive
micro-electro-mechanical systems, or MEMS, can already be etched
from silicon, including gears, pumps, electric motors and even
complex devices like microscopic steam engines. Unfortunately,
today's MEMS don't last long. They're made of silicon because the
electronics industry has given us a number of advanced techniques
for shaping that particular substance on the micro scale. But
silicon is highly susceptible to an atomic friction force called "stiction,"
which tends to jam and erode very small moving parts. If this
problem can be overcome, or alternative materials found, the
technology to build a primitive foglet could be in our hands within
a decade.
Still, the journey from foglet to
utility fog could be a long one indeed. There'd be enormous
challenges in communication, power distribution and especially the
intelligent coordination of activity between thousands or millions
of foglets. Not to mention getting their little arms to grab
reliably, without breaking off.
Fortunately, silicon offers another
exciting possibility in its role as a semiconductor. Most materials
are either conductors, which permit the free flow of electrons, or
insulators, which don't. Semiconductors are insulators which are
capable of conducting electrons within a certain narrow energy
band--a very useful property in the field of electronics.
Silicon's electrical properties are
fixed by the laws of physics, but through "doping," the carefully
controlled introduction of impurities, its crystals can be tuned so
that, for example, room-temperature electrons have a good chance of
jumping up into the conduction band when a voltage is applied.
Silicon doped with electron donor atoms such as phosphorus becomes
an "N" or negative-type semiconductor, through which electrons can
travel more easily. Doping with electron borrowers like aluminum
produces a "P" or positive material, which conducts "holes," or
spaces where an electron isn't. A "P" layer adjacent to an "N" layer
creates a P-N junction, a kind of electrical "valve" or "gate" which
permits electrons to flow easily in one direction but not the other.
This effect is used in electronic
components such as diodes, light-emitting diodes and transistors,
but from a programmable matter standpoint the most interesting
applications occur when an N layer is sandwiched between two Ps.
This creates a kind of "trap" which attracts electrons into the
middle layer and doesn't let them out. This is a useful trick all by
itself, but if the P layer is thin enough--about 10 nanometers or
0.000001 millimeters or 50 atoms high--we enter the realm of quantum
mechanics, and the electrons trapped in the P layer begin to do
something strange and potentially wonderful: along the vertical
dimension they start behaving as waves rather than particles.
Such devices, known as quantum wells,
are easy and cheap to produce, and find practical use in computers,
fiber-optic networks and those $10 keychain laser pointers everyone
seems to have these days. But even that isn't the exciting part. A
quantum well confines electrons in a two-dimensional layer, like the
meat inside a sandwich. But if the meat and top bread layers are
sliced away, leaving a narrow stripe of P-N sandwich on top of a
sheet of P bread, the electrons take on wavelike behavior along an
additional axis. This structure is called a "quantum wire." Finally,
etching away the sides of the stripe to leave a tiny square of meat
and bread resting on the lower slice, we can produce a "quantum dot"
(as seen below) which confines the electrons in all three
dimensions, forcing them to behave as standing waves, or probability
density functions, or strangely shaped clouds of diffuse electric
charge.
Quantum dots could create Camelot

There is one other place where
electrons are known to behave this way: in atoms. Electrons which
are part of an atom will arrange themselves into negatively charged
"orbitals" which constrain and define their positions around the
positively charged nucleus. These orbitals, and the electrons which
partially or completely fill them, are what determine the chemical
properties of an atom, i.e., what other sorts of atoms it can bind
to, and how strongly.
The thing that's wonderful and
important and shocking about a quantum dot is that the electrons
trapped in it will arrange themselves just as though they were part
of an atom, even though there's no atomic nucleus for them to
surround. Which atom they emulate depends on the number of excess
electrons trapped inside. What's more, the electrons in two adjacent
quantum dots will interact just as they would in two real atoms
placed at the same distance, meaning the two dots can share
electrons between them, meaning they can form genuine chemical
bonds.
Now we'll take it a step further:
quantum dots needn't be formed by etching squares out of a quantum
well. Since like charges repel one another (and opposites attract),
electrons in the well can instead be confined by rings or squares of
negatively charged material on the upper "P" layer, a kind of
electrostatic fence or corral atop the quantum well sandwich. This
is better because it lets us adjust the quantum dot's
characteristics simply by varying the voltage on the fence. Thus, we
can not only form "chemical" bonds between dots, but also turn the
bonds on and off as electrons are pumped in and out.
That's virtual chemistry, baby!
Programmed changes in shape and texture are all well and fine, but
not really so different from what we can achieve manually, with a
machine shop or even a simple potter's wheel. But here is something
entirely new: a material capable of changing its very substance.
And that's not all; quantum dots can also be made in various shapes,
offering possibilities beyond the symmetries of normal atoms. And
where cranky nuclear forces limit the number of electrons in a
stable atom to 92, quantum dots can hold many times that
number--electrons by the hundreds, forming gigantic new orbitals
classical chemists could never have imagined.
In short, these controllable quantum
dots--what Yale University's Mark Reed calls "designer atoms"--can
not only mimic every atom on the periodic table, but can quite
easily produce atom-like structures with properties that don't occur
in nature. For example, with many more electrons to share than even
the faithful carbon atom, such designer atoms may be capable of
forming superstrong chemical bonds, to produce materials much
tougher than diamond, the hardest natural substance. Other
possibilities include dramatic changes in the reflection and
absorption of light, and the conduction of electricity. (Just for
starters, think of an indestructible, 100% efficient solar energy
cell.)
So picture this: a lattice of
crystalline silicon, superfine threads much thinner than a human
hair crisscrossing to form a translucent structure like microscopic
basket wicker. Except that with the application of electrical
currents, the spaces between the threads can be filled with "atoms"
of any desired species, producing a virtual substance with the mass
of wickered silicon, but the chemical, physical and electrical
properties of some new, hybrid material. And changeable at the flip
of a bit, to a nearly infinite variety of other materials!
In contrast to the biology-like
limitations of nanotechnology and the fragile complexity of utility
fog, such a material (which I have elsewhere dubbed "wellstone")
would be capable of dramatic and instantaneous changes more akin to
magic than to technology as we've previously known it. So watch out:
if this trick does turn out to be technologically feasible, then the
high-tech, high-gloss future of Star Trek may slip from our
fingers, to be supplanted by something more like the mists and
dreams and enchantments of Camelot. Or even--God help us all--Olympus!
Wil McCarthy is a rocket guidance
engineer, robot designer, science fiction author and occasional
aquanaut. He has contributed to three interplanetary spacecraft,
five communication and weather satellites, a line of
landmine-clearing robots, and some other "really cool stuff" he
can't tell us about. His short fiction has graced the pages of
Analog, Asimov's, Science Fiction Age and other
major publications, and his novel-length works include Aggressor
Six, the New York Times notable Bloom, and The
Collapsium.
|